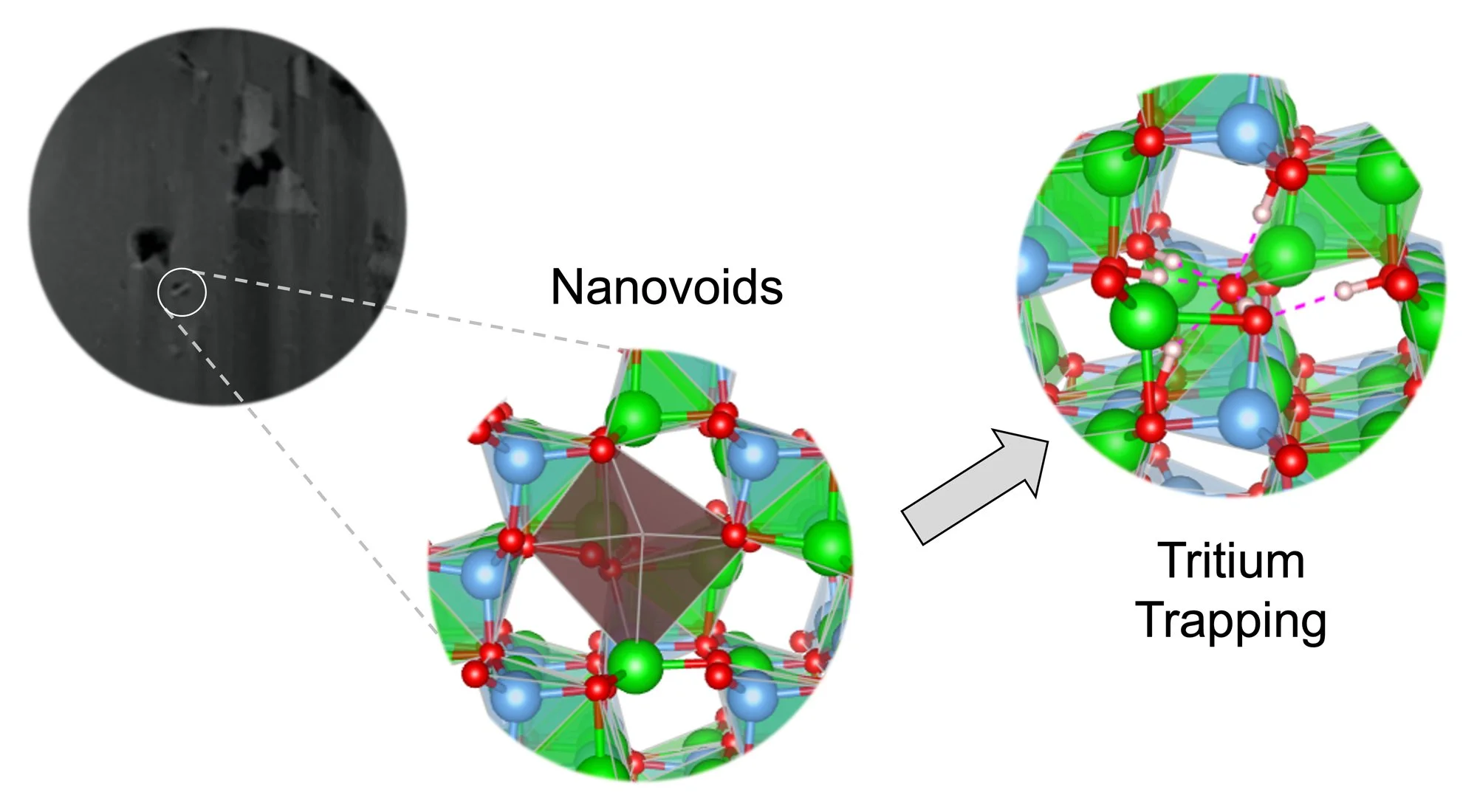
Paper on Tritium Trapping in LiAlO2 Nanovoids Published in JPCC
Our team’s latest paper on first principles modeling of tritium trapping in neutron-irradiated γ-LiAlO2 has just been published in the Journal of Physical Chemistry C! Led by Michel Sassi, this study describes first principles models for tritium behavior informed by analytical transmission electron microscopy. We elucidate the presence and role of voids in this system, with implications for nuclear energy.
From the abstract:
Production of radioactive tritium species is efficiently performed in nuclear reactors by neutron irradiation of Tritium Producing Burnable Absorber Rods (TPBARs). In TPBARs, the essential components for tritium production are 6Li-enriched γ-LiAlO2 pellets. Post-irradiation experimental analysis identified irradiation induced microstructures at the nanoscale level, among them, vacancy clusters and voids which are present in γ-LiAlO2 pellets, potentially affecting the diffusion of tritium out of the pellets. In this work, scanning transmission electron microscopy (STEM) provides additional experimental evidence for the presence of nanovoids in neutron irradiated pellets and first principles ab initio thermodynamics simulations were carried out to evaluate the energetics of tritium trapping by single and double cation and anion vacancies as function of temperature and 𝑝O . The results indicate that tritium trapping allows recovery of an important amount of the formation energy cost of a cation vacancy, while the trapping of tritium by anion vacancies further increases the energy cost of forming a vacancy. The simulations also indicated the potential for tritium to be trapped in various forms by forming bonds such as Al—T, O—T or T2. A Bader charge analysis provided additional details about the charge of the tritium species trapped in vacancy space, such that T+ species are trapped in cation vacancies while T– species are trapped in anion vacancies. This further indicates that tritium bonded to O atoms are positively charged, while those bonded to Al atoms are negatively charged.
To view the manuscript, visit: https://doi.org/10.1021/acs.jpcc.2c00381
To download the article directly, click here.