LSNFO OER Paper Published in Nanoscale
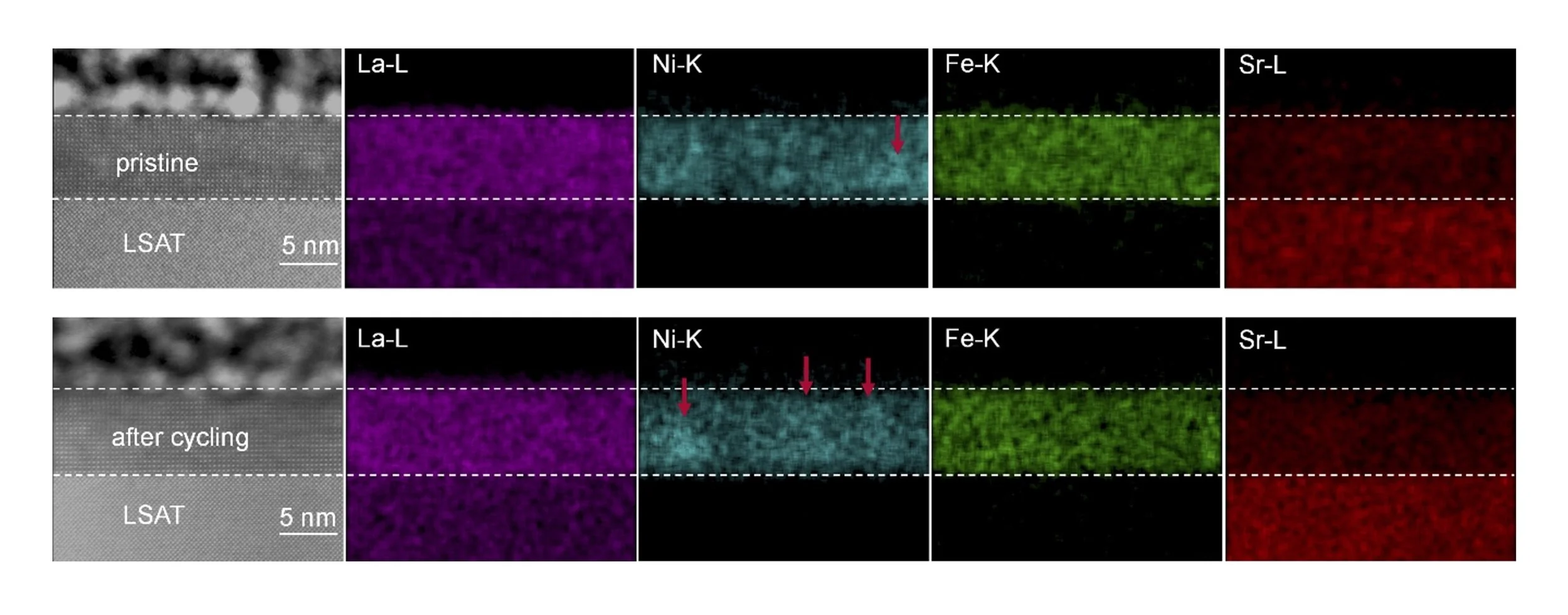
Understanding oxygen evolution reaction (OER) behavior at oxide surfaces is critical for emerging clean energy applications. In our recent study led by Prajwal Adiga and Kelsey Stoerzinger at OSU and Le Wang at PNNL, we explore a promising class of model Fe and Ni-containing oxide thin films. Writing in the journal Nanoscale, we observe the evolution of surface composition during electrochemical cycling, identifying common motifs of the active surface with high surface area systems. This information will help us in the rational design of improved catalytic surfaces.
From the abstract:
Water electrolysis can use renewable electricity to produce green hydrogen, a portable fuel and sustainable chemical precursor. Improving electrolyzer efficiency hinges on the activity of the oxygen evolution reaction (OER) catalyst. Earth-abundant, ABO3-type perovskite oxides offer great compositional, structural, and electronic tunability, with previous studies showing compositional substitution can increase the OER activity drastically. However, the relationship between the tailored bulk composition and that of the surface, where OER occurs, remains unclear. Here, we study the effects of electrochemical cycling on the OER activity of La0.5Sr0.5Ni1-xFexO3-δ (x = 0-0.5) epitaxial films grown by oxide molecular beam epitaxy as a model Sr-containing perovskite oxide. Electrochemical testing and surface-sensitive spectroscopic analyses show Ni segregation, which is affected by electrochemical history, along with surface amorphization, coupled with changes in OER activity. Our findings highlight the importance of surface composition and electrochemical cycling conditions in understanding OER performance, suggesting common motifs of the active surface with high surface area systems.
To download the paper, visit the publisher website.